Original link: http://tecdat.cn/?p=24721
In this paper, I use two examples that may be of interest to population ecologists to illustrate the use of "JAGS" to simulate data: the first is linear regression, and the second is to estimate animal survival rate (formulated as state space model).
Recently, I have been trying to simulate data from complex hierarchical models. I'm using JAGS now.
Simulation data {JAGS} is convenient because you can use (almost) the same code for simulation and reasoning, and you can conduct simulation research (deviation, accuracy, interval coverage) in the same environment (i.e. JAGS).
Linear regression example
Let's first load the packages required for this tutorial:
library(R2jags)
Then let's get straight to the point and let's generate data from the linear regression model. Use a data block and pass parameters as data.
data{
#Likelihood function:
for (i in 1:N){
y\[i\] ~ #tau is precision (1 / variance).
}Here, alpha and beta are intercept and slope, precision or reciprocal of tau variance, y dependent variable and x explanatory variable.
We choose some values for the model parameters used as data:
#Simulated parameters N #Sample x <- 1:N #Predictor alpha #Intercept beta #Slope sigma#Residual sd 1/(sigma*sigma) #Precision #In the simulation step, the parameters are treated as data
Now run JAGS; Please note that we monitor dependent variables instead of parameters, as we do in standard reasoning:
#Operation results out

The output is a little messy and needs to be properly formatted:
#Reformat output mcmc(out)
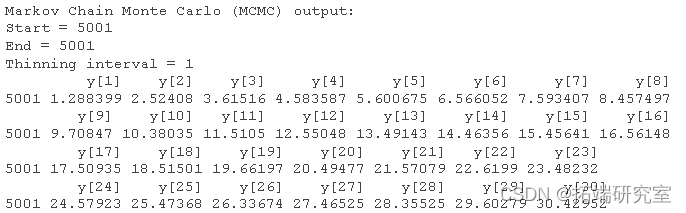
dim
dat

Now let's fit the model we used to simulate into the data we just generated. It is assumed that the reader is familiar with , JAGS , linear regression.
#Specifying models in BUGS language
model <-
for (i in 1:N){
y\[i\] ~ dnorm(mu\[i\], tau) #tau is precision (1 / variance)
alpha intercept
beta #Slope
sigma #Standard deviation
#Data
dta <- list(y = dt, N = length(at), x = x)
#Initial value
inits
#MCMC settings
ni <- 10000
#Calling JAGS from R
jags()
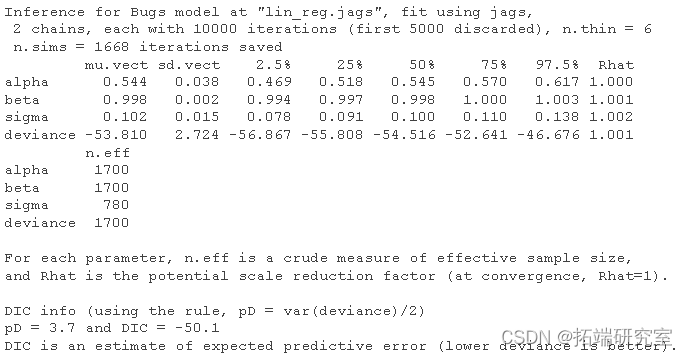
Let's look at the results and compare them with the parameters we used to simulate the data (see above):
#Summary posteriori print(res)
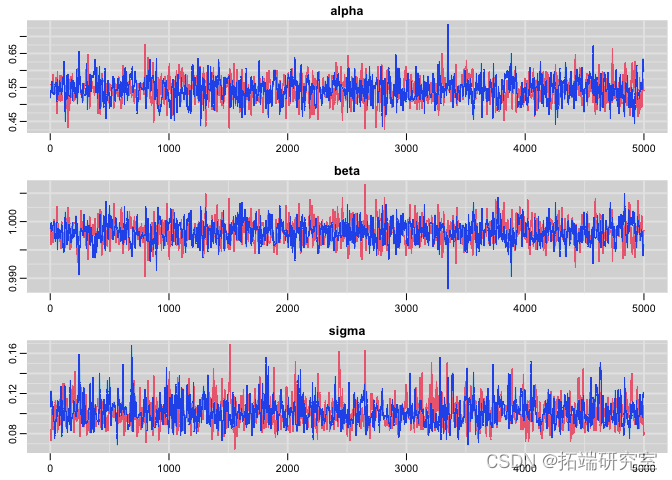
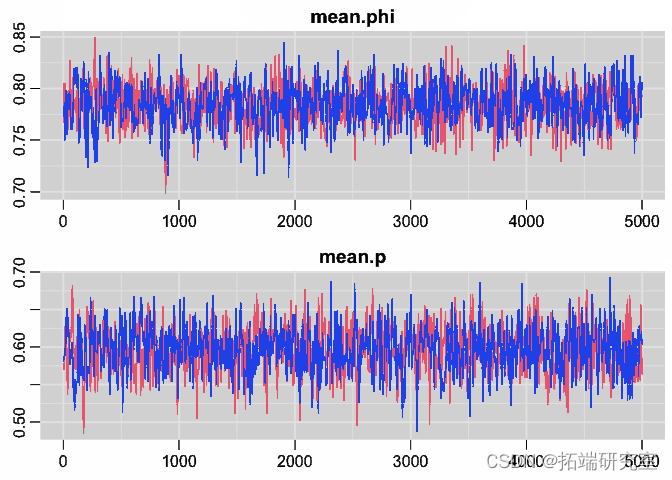
Check Convergence:
#Tracking chart plot(res)
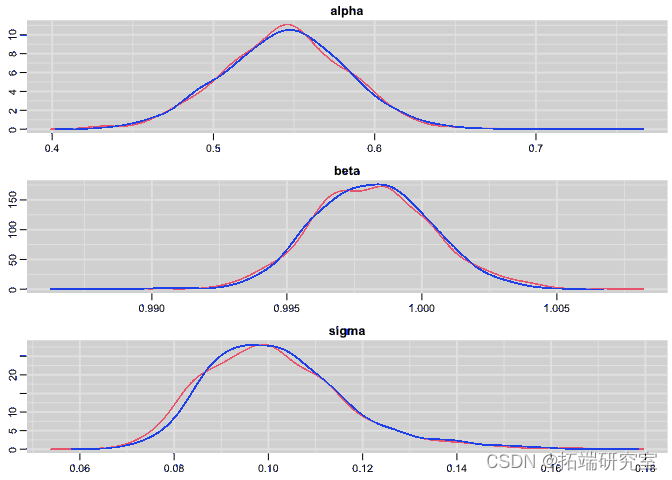
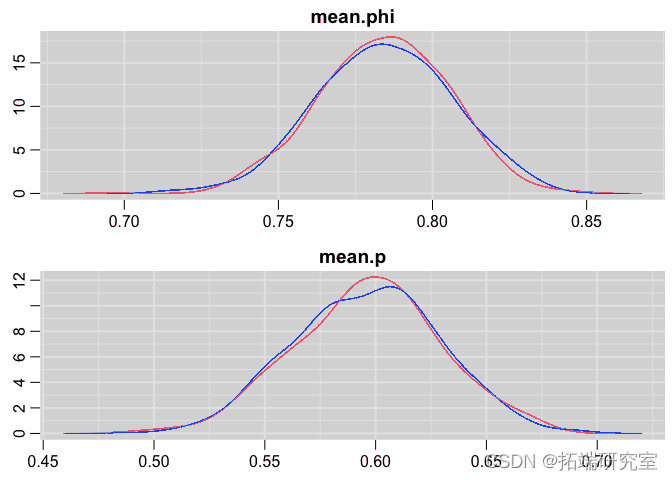
Plot the posterior distribution of regression parameters and residual standard deviation:
#Posterior distribution plot(res)
Simulation example
I will now explain how to use JAGS to simulate data from models with constant survival and recapture probabilities. I assume that the reader is familiar with this model and its formula as a state space model.
Let's simulate it!
#Constant survival and recapture probability
for (i in 1:nd){
for (t in f:(on-1)){
#probability
for (i in 1:nid){
#Define latency and observations at first capture
z\[i,f\[i\] <- 1
mu2\[i,1\] <- 1 * z\[i,f\[i\] #It is detected as 1 at the first capture ("subject to the first capture").
#Then deal with the future situation
for (t in (f\[i\]+1):non){
#Status process
mu1\[i,t\] <- phi * z
#Observation process
mu2\[i,t\] <- p * zLet's select some values for the parameters and store them in the data list:
#Parameters for simulation n = 100 #Number of individuals meanhi <- 0.8 #Survival rate meap <- 0.6 #Recapture rate data<-list
Now run JAGS:
out

Format output:
as.mcmc(out)
head(dat)

I only monitor the detection and non detection, but I can also obtain the simulation value of the state, that is, whether the individual is alive or dead in each case. You just need to modify the call to JAGS , monitor=c("y","x") and modify the output accordingly.
Now we fit the Cormack jolly Seber (CJS) model to the data we just simulated, assuming that the parameters remain unchanged:
#Tendencies and constraints
for (i in 1:nd){
for (t in f\[i\]:(nn-1)){
mehi ~ dunif(0, 1) #A priori value of mean survival
Me ~ dunif(0, 1) #A priori value of average recapture
#Probability
for (i in 1:nd){
#Defines the latency at the first capture
z\[i\]\] <- 1
for (t in (f\[i\]+1):nions){
#State process
z\[i,t\] ~ dbern(mu1\[i,t\])
#Observation process
y\[i,t\] ~ dbern(mu2\[i,t\])Prepare data:
#Vector of marked occasion gerst <- function(x) min(which(x!=0)) #Data jagta
#Initial value
for (i in 1:dim\]){
min(which(ch\[i,\]==1))
max(which(ch\[i,\]==1))
function(){list(meaphi, mep , z ) }We want to infer the probability of survival and recapture:
Standard MCMC settings:
ni <- 10000
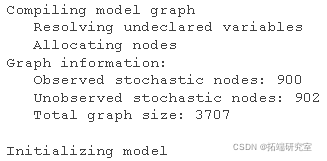
Ready to run JAGS!
#Calling JAGS from R jags(nin = nb, woy = getwd() )
Summarize the posteriori and compare it with the values we used to simulate the data:
print(cj3)
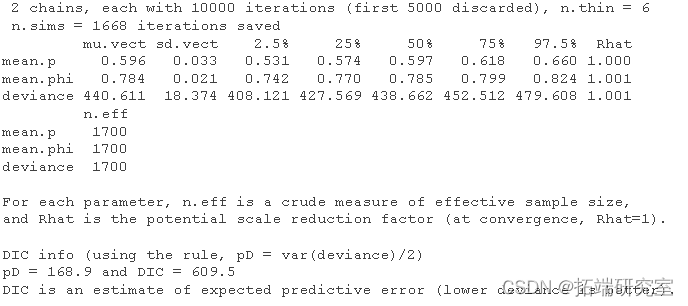
Very close!
Tracking chart
trplot
Posterior distribution map
denplot

Most popular insights
1.Deep learning using Bayesian Optimization in matlab
2.Implementation of Bayesian hidden Markov hmm model in matlab
3.Bayesian simple linear regression simulation of Gibbs sampling in R language
4.block Gibbs Gibbs sampling Bayesian multiple linear regression in R language
5.Bayesian model of MCMC sampling for Stan probability programming in R language
6.Python implements Bayesian linear regression model with PyMC3
7.R language uses Bayesian hierarchical model for spatial data analysis
9.Implementation of Bayesian hidden Markov hmm model in matlab